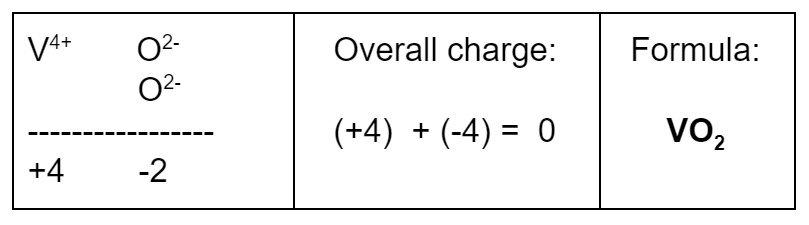(See: Binary Ionic Componts Part 1)
You can predict the formula for ionic compounds if you know the charge of each element.
Rule: Compounds are neutral. They have an overall charge of zero.
Example: What’s the formula of a compound with K+ and O2−?
Answer: K2O
To get a total of zero charge, we need a 2:1 ratio.
We need two K+ ions for every one O2− ion.
Here’s the charge math:
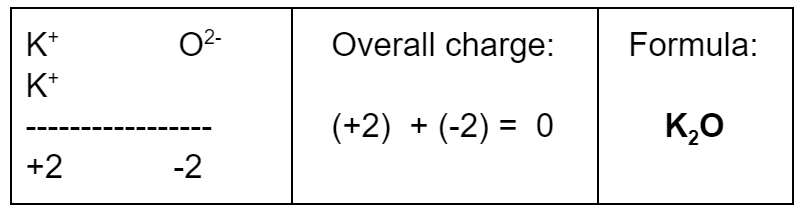
Things to notice:
- The positive ion is written first in the formula.
- The “2” subscript in K2O means there are two K+ ions.
- Superscripts (small raised numbers) show an ion’s charge.
- Superscripts (charge) are not written in the compound’s formula.
Next Example: 2’s and 3’s
What is the formula of a compound made of Ru3+ and O2−?
-
- You need the common denominator for 2 and 3, which is 6. Make the positive and negative charges both add up to 6, and then the overall charge will be zero.
- To get an overall charge of zero, you need two Ru3+ ions for every three O2− ions.
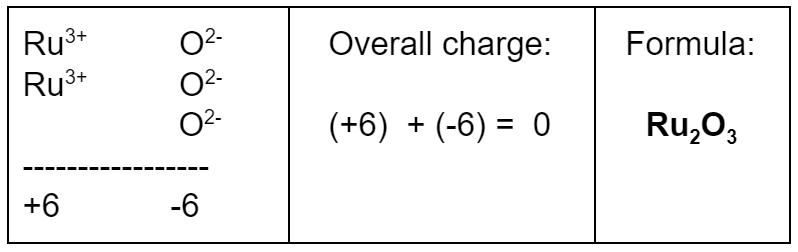
Tip: you can “cross the charges” when you do problems like this. Notice that when you write the ions with their charges (blue numbers), the 2 and 3 switch places. If you use this trick, don’t ever make a subscript negative – you can’t have a negative number of atoms in a molecule.
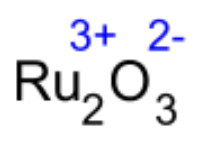
Last Example: Use Simplest Ratio
What is the formula of a compound made of V4+ and O2−?
- The common denominator of 2 and 4 is 4. Therefore, include enough of each ion to get positive and negative charges of 4. This way, the overall charge will be +4 – 4 = 0.
- If you “cross the charges” by switching 2 and 4, as in the tip for Example 3, you’ll come up with a formula of V2O4, but that is a wrong answer – it needs to be simplified to VO2. Use the simplest whole number ratio.
- To get an overall charge of zero, you need one V4+ ion for every two O2− ions.