Chemical reaction math only works when your amounts are in moles. This is because whole number ratios of molecules react in a chemical reaction. A mole is a certain number of molecules, so the ratios still work when your amounts are in moles. The ratios from balanced equations don’t work when your amounts are in grams (mass units).
Molar Mass
This is the mass of one mole of a substance. Units are ![]() .
.
Look up the molar masses of elements on the Periodic Table.
For compounds, add up the molar masses of all the atoms.
Example 1: calculate molar mass of NO2
Solution 1:
Step 1: Find the number of atoms of each element.
Step 2: Multiply number of atoms by molar mass for each element.
Step 3: Add up the total masses of the elements.
| Element | # of atoms | element mass | # of atoms times mass |
| N | 1 | 14.01 g/mol | 14.01 g/mol |
| O | 2 | 16.00 g/mol | 32.00 g/mol |
| Total of all elements | 46.01 g/mol |
Answer: molar mass of NO2 is ![]()
Converting Moles to Grams
Multiply your amount of moles by the molar mass to convert the amount to grams.
Example 2: What is the mass (in grams) of 3.75 moles of aluminum?
Solution 2 using Dimensional Analysis:
Using the Periodic Table, the molar mass of aluminum is 26.98 g/mol.
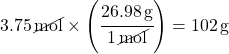
Converting Grams to Moles
Divide your amount in grams by the molar mass. This is the same as multiplying by the reciprocal of the molar mass.
Example 3: How many moles is 208 grams of aluminum?
Solution 3 using Dimensional Analysis:
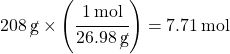
Notice that the molar mass fraction is flipped (reciprocal) this time, with grams on the bottom and moles on top. This is so grams will cancel out and give a result in moles. Since the 26.98 is on the bottom of the fraction, you divide by that number to get the answer.