- Ions form when atoms gain or lose electrons.
- Ions have a non-zero charge.
An atom of fluorine has equal numbers of protons (9) and electrons (also 9). It is neutral. Its overall charge is zero:
| proton charges: | +9 |
| electron charges: | -9 |
| overall charge: | 0 (neutral) |
Let’s look at happens when a neutral fluorine atom becomes an ion.
Neutral fluorine’s electron arrangement looks like this:
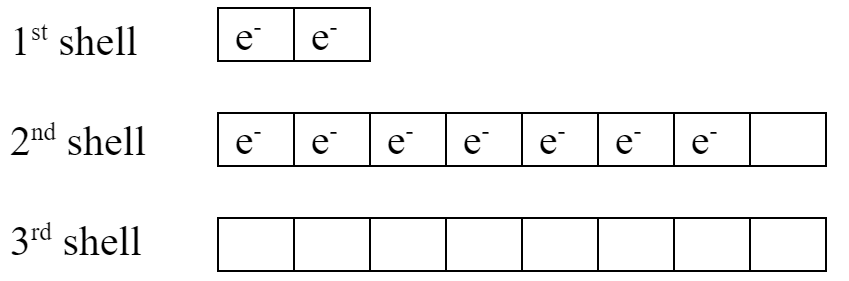
Fluorine has room to add one more electron in its 2nd shell. It will gain an electron when it becomes an ion and fill that shell. It’s not strong enough to attract any electrons to its 3rd shell.
A fluorine ion has a filled 2nd shell and an overall -1 charge:
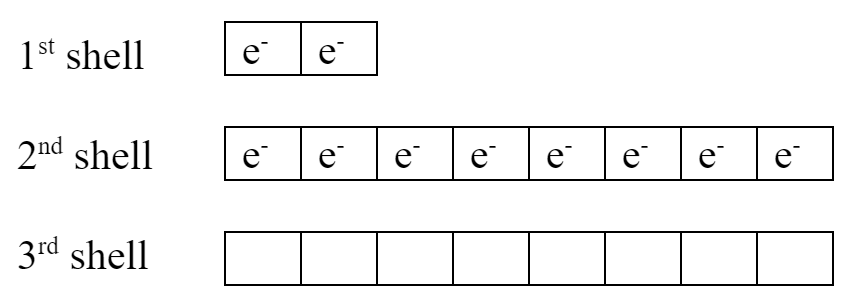
This ion has a filled valence shell. Its electron arrangement is exactly the same as a neon atom. Neon is a noble gas – noble gases have full valence shells. It’s very common for elements to gain or lose electrons and end up with a full valence shell like a noble gas.
Now its charge situation looks like this:
| proton charges: | +9 |
| electron charges: | -10 |
| overall charge: | -1 |
Many nonmetals follow this pattern: they gain enough electrons to fill their valence (highest occupied) electron shell. Their charge is determined by the number of electrons required to fill that shell.
Metals behave in the opposite way: they lose electrons to empty out their valence shells.
Example: neutral sodium atom has one valence electron in 3rd shell:
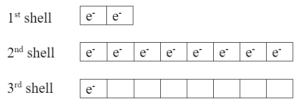
When it becomes an ion, it loses that one electron and ends up with a +1 charge. Sodium ions are positive because they have more protons (11) than electrons (10).
| proton charges: | +11 |
| electron charges: | -10 |
| overall charge: | +1 |
Both sodium ions and fluorine ions end up with the same electron configuration, which is also the same as an atom of neutral neon (a noble gas). This is no coincidence! Noble gas electron configurations are common, stable arrangements, because elements are often able to pull strongly enough to gain electrons to fill their valence shells, yet too weak to gain additional electrons that would have to be in the next higher shell, farther away and more weakly held by the nucleus.