Chemical reaction calculations don’t work using mass units (grams), so you need to convert your amounts to moles first. Usually you need an answer in grams, so you convert back to mass units at the end.
Example Problem
How many grams of chlorine gas (Cl2) are needed to produce 38.5 grams of NCl3?
![]()
Solution
- Convert the known mass to moles.
You will always be told a known amount of one of the substances. Start with this known amount: 38.5 g NCl3
To convert to moles, you first need the molar mass of NCl3.
Use the Periodic Table to look up the masses of N and Cl and add up the molar mass:
N: 14.01 x 1 = 14.01
Cl: 35.45 x 3 = 106.35
molar mass = total = 120.36 g/mol
Divide mass by molar mass to calculate moles.
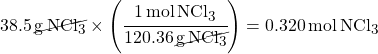
(This is using Dimensional Analysis to cancel out units)
2. Multiple by a Mole Ratio to find moles of the unknown substance.
This problem asks you to calculate grams of Cl2. First you have to calculate moles of Cl2 using a mole ratio. The balanced equation coefficients give you the mole ratio.
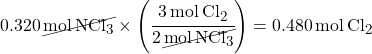
3. Convert moles to grams.
First you need molar mass of Cl2, so multiply the mass of Cl from the Periodic Table by two:
35.45 x 2 = 70.9 g/mol
Multiply moles of Cl2 by molar mass of Cl2 to convert the amount to mass units (grams):
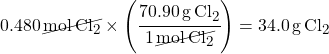
This is what the problem asked for: mass of chlorine gas.