These pictures show how three different element properties change as you move around the Periodic Table.
Definitions
Atomic Radius: distance to an atom’s valence (outer) electrons.
Ionization Energy: amount of energy needed to remove a valence electron from an atom.
Electronegativity: Ability to attract (steal) electrons from neighboring atoms.
Atomic Radius going down a Group
Which of the Alkaline Earths (Group 2) has the biggest atomic radius?
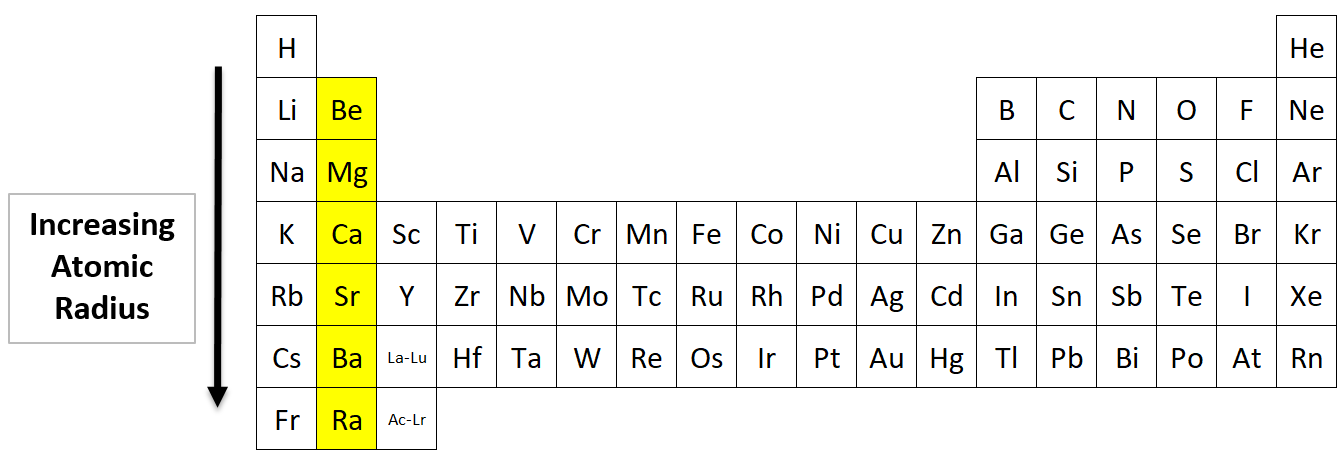
Answer: Radium (Ra) is the largest. The elements at the bottom have the most electron shells, so they have a bigger radius.
Atomic Radius going across a Period from left to right
Which Period 3 element has the largest atomic radius?
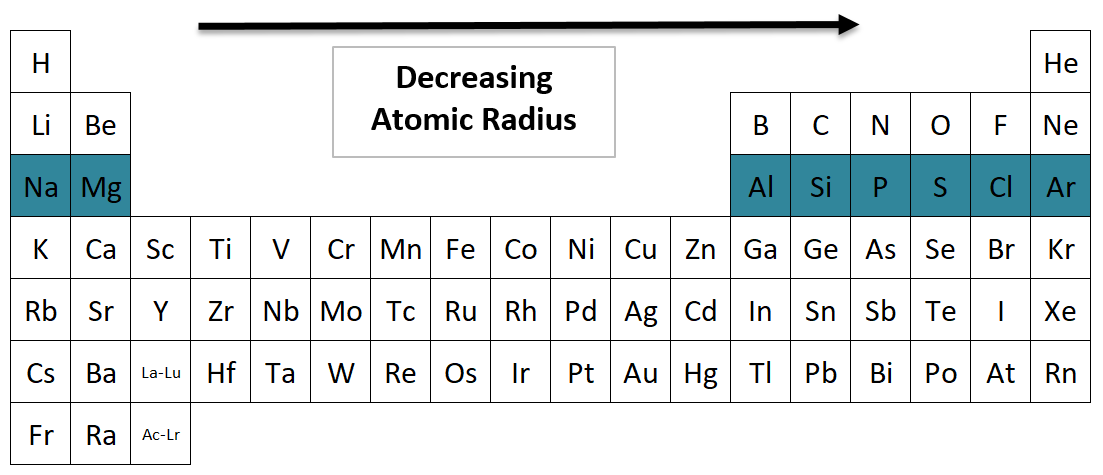
Sodium (Na) has the largest atomic radius in Period 3. The elements get smaller as you go to the right, because they have more protons to pull the electron cloud closer to the nucleus.
Ionization Energy going down a Group
Which element in Nitrogen’s family (Group 15) has the highest ionization energy?
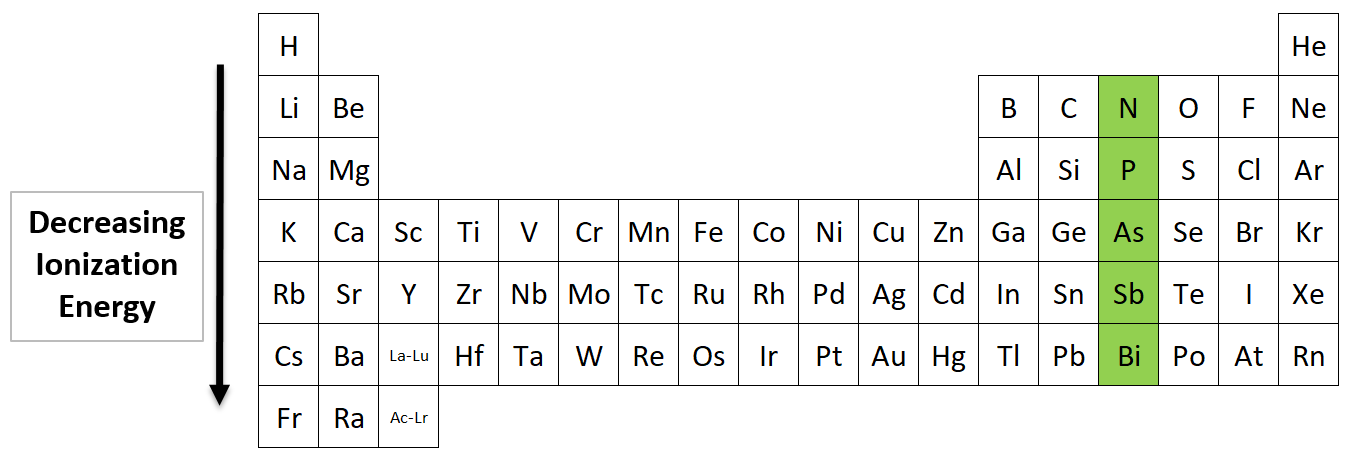
Nitrogen (N) has the highest ionization energy in its family. As you go down the table, the elements have more electron shells, which means their valence shells are farther from the nucleus. Electrons farther from the nucleus are easier to remove.
Ionization Energy going across a Period from left to right
Which Period 2 element has the highest ionization energy?
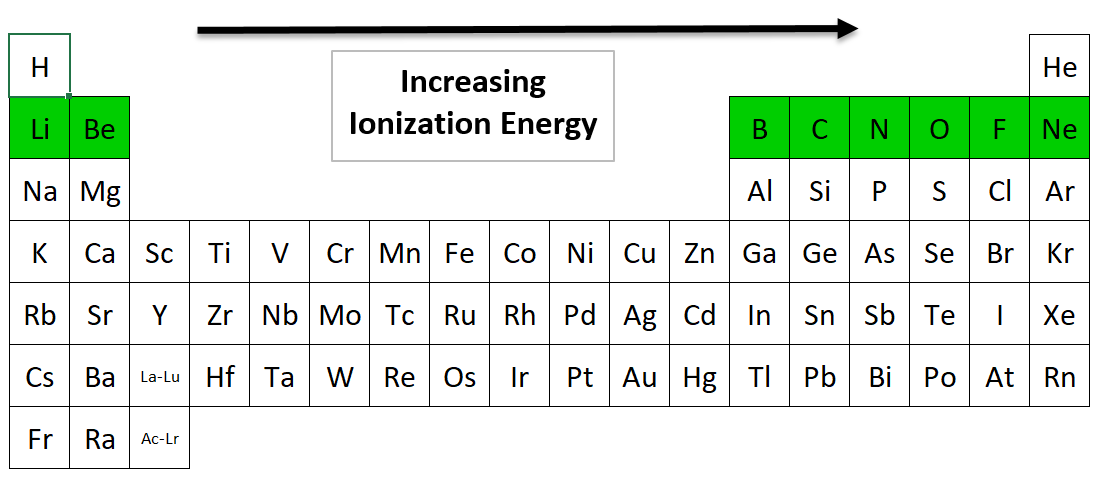
Neon (Ne) has the highest ionization energy in Period 2. As you move to the right, the elements have more protons. These extra protons make it harder to remove an electron from the outer shell. It requires more energy.
Electronegativity going down a Group
Which halogen (Group 17) has the highest electronegativity?
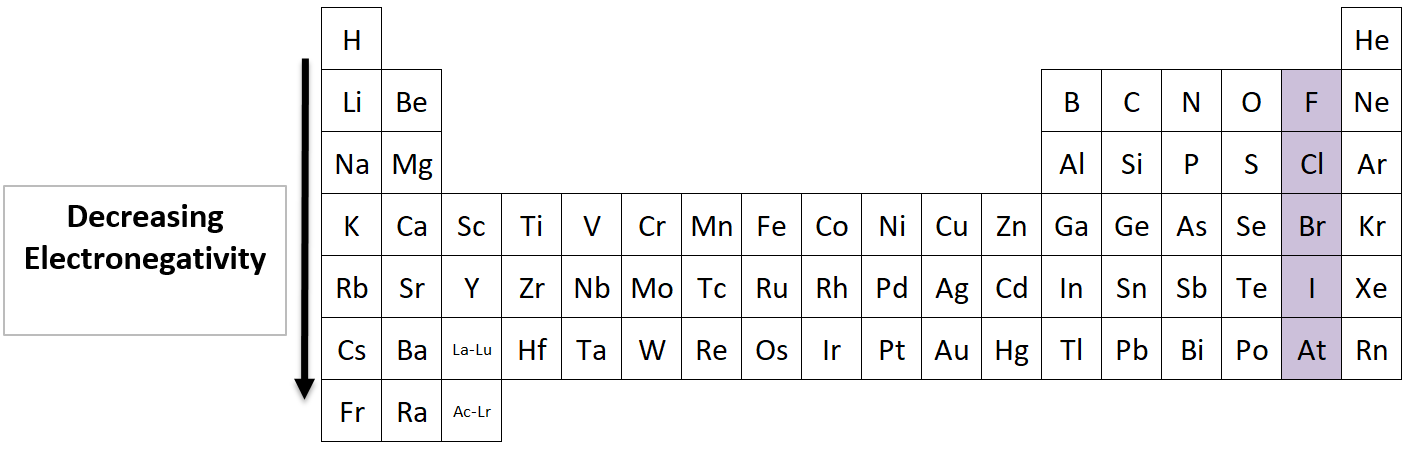
Fluorine has the highest electronegativity in its family. Electronegativity decreases as you move down the table. The elements at the bottom have more electron shells, which means their nucleus is farther away from a neighboring atom’s electrons.
(bonus: Fluorine has the highest electronegativity of any element)
Electronegativity going across a Period from left to right
Which Period 4 element has the highest electronegativity?
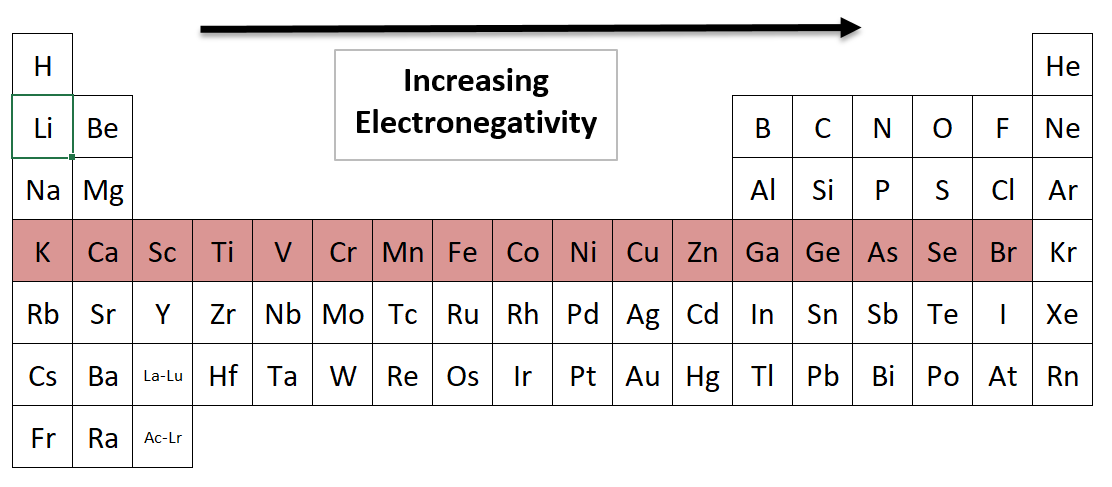
*Noble gases don’t have normal electronegativity values, so we are ignoring Krypton (Kr). It doesn’t attract electrons from other atoms. It has nowhere to put another electron, because its valence shell is full.
Bromine (Br) has the highest electronegativity in Period 4. It is the furthest to the right (other than Krypton, a noble gas). Elements have more protons as you move to the right, and that gives them a stronger pull to attract electrons from neighboring elements.