You can use a Periodic Table to find the charge that an element is likely to have as an ion.
Elements’ position on the table tells you their valence electrons, and that determines what charge would give them full valence shells as as ions. Metals tend to lose all of their valence electrons, and nonmetals tend to gain enough electrons to get to 8 valence electrons for a full shell. (Disclosure: there are many exceptions to this behavior)
Examples (see Element Families notes if needed):
- Alkali metals all have one valence electron, which they lose to form ions with +1 charge. Example: sodium (Na) forms Na+ ions.
- Alkaline earths have two valence electrons, which they lose to form ions with +2 charge.
- Halogens have 7 valence electrons, and they gain one more electron, filling their valence shells to form ions with -1 charge.
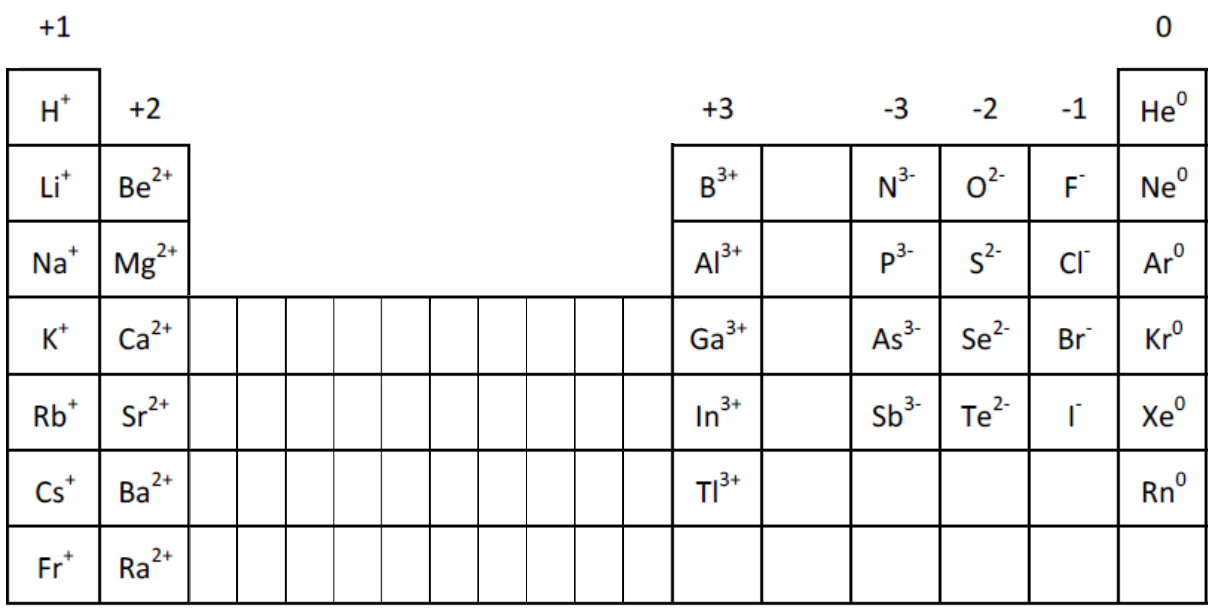
(The numbers at the top of the columns show what charge that family’s elements sometimes have as ions.)
Bonus Content (more advanced):
-
- These elements don’t always follow such this pattern. For example, phosphorus is actually more likely to be found with a positive charge in a compound with oxygen. But at least we can say that it’s capable of forming a P3- ion to fit the pattern shown in the chart.
- The transition metal elements (middle section of the table) don’t have the same type of predictable charge patterns, and most of them are capable of forming more than one type of ion. For example, iron can form Fe2+ and Fe3+ ions, depending on the situation.
- The elements in the carbon family aren’t listed as +4 or -4 charged ions, because they are more likely to form covalent bonds instead of being ions. But it’s possible for carbon and silicon to form a C4- / Si4- ions, and all of that family’s elements can form +4 ions. Therefore, sometimes that row is thought of as having “+/- 4” charge, which fits nicely between the +3 and -3 columns.
- You can check out this fancier Periodic Table to see the most common charges of most of the elements.
- Most elements have the ability to form more than one charge, but there are patterns to be found there, as well. For example, metals often lose enough electrons to leave two valence electrons remaining (example: carbon family with +2 charge). This gives them a full S subshell, which is a pretty stable configuration. Read about electron configurations to learn more.