Alkanes: the simplest hydrocarbons.
•only made of carbon and hydrogen
•only has single bonds
The chemical formula of alkanes follows this pattern:
CnH2n+2
Example:
•Let’s say a certain alkane has 4 carbon atoms (C) , so n=4.
•If n=4, then 2n+2 = 10.
•2n+2 is the number of hydrogen atoms (H), so there are 10 H’s.
•Therefore, the formula for a 4 carbon alkane is C4H10.
Alkane Names
Alkanes always end with the suffix “-ane.”
They start with a prefix that indicates the number of carbon atoms:
| # of C’s | Prefix |
| 1 | meth- |
| 2 | eth- |
| 3 | prop- |
| 4 | but- |
| 5 | pent- |
| 6 | hex- |
| 7 | hept- |
| 8 | oct- |
| 9 | non- |
| 10 | dec- |
Example: what is the name of a 6 carbon alkane? Answer: hexane.
Drawing / Building Alkanes;
•Carbon always has exactly four bonds (8 electrons)
•Hydrogen always has exactly one bond (2 electrons)
Hydrocarbons can be drawn in multiple ways. The drawings below all represent the same molecule: pentane (C5H10), a five carbon alkane.
Structural Formula
Each dash in the diagram represents one chemical bond, and each bond is made up of two electrons.
![Rendered by QuickLaTeX.com \definesubmol{c}{-C(-[2]H)(-[6]H)} \chemfig{[,.6]H!c!c!c!c!c-H}](https://www.nemoquiz.com/wp-content/ql-cache/quicklatex.com-d2136a9f13bc78beca288871b8a0a241_l3.png)
Notice that each carbon atom is surrounded by four bonds, and each hydrogen has one bond.
Often structural formulas are drawn without any hydrogen atoms. This saves writing and makes it easier to see the carbon backbone of the molecule.
![]()
Electron Dot Diagram (also known as Lewis Structure)

Each pair of dots represents a covalent bond (electron pair).
Notice that each C atom has 8 electrons around it (full 2nd electron shell) and each H atom has 2 electrons (full 1st electron shell).
8 electrons = 4 electron pairs = 4 bonds
Molecular Formula or Chemical Formula
Pentane’s molecular formula is ![]()
Pentane can also be written out like this:
![]()
Bonus: Stick Diagram
Even though it’s very simple, the following is another way to represent pentane:
*** QuickLaTeX cannot compile formula:
\definesubmol{a}{-[:30]-}
\definesubmol{b}{-[:30]=[0]-}
\chemfig{[:-30,0.6]-!a!a!}
*** Error message:
Package chemfig Error: no submol name found after "!"..
leading text: \chemfig{[:-30,0.6]-!a!a!}
Undefined control sequence \_nil.
leading text: \chemfig{[:-30,0.6]-!a!a!}
Missing $ inserted.
Emergency stop.
•Each vertex (point) is a carbon atom. Elements other than carbon and hydrogen are shown with their normal element symbol.
•Hydrogen atoms connected to carbons are not shown. And unlike structural formulas, the bonds to hydrogen aren’t shown, either. Chemists don’t need that information, because they know carbons have four bonds, so they can figure out how many H’s are attached to each carbon atom.
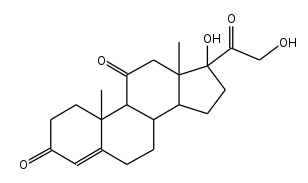
Chemists typically use stick diagrams to show large organic molecules, because they look cleaner.
Example: this stick diagram of cortisone would look pretty messy if it had all of the hydrogen atoms included.