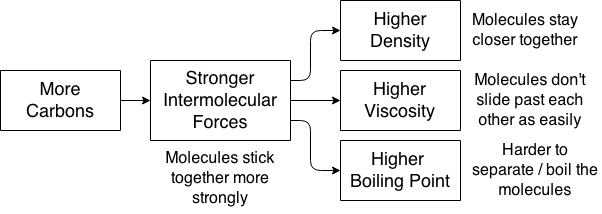
Viscosity: resistance to flow. Liquids with high viscosity, like honey, are commonly called “thick.”
Density: ![]() . Ratio of mass to volume.
. Ratio of mass to volume.
Intermolecular Forces: forces between molecules. Weaker than chemical bonds.
Chemical Bonds: forces within molecules. Typically much stronger than intermolecular forces.
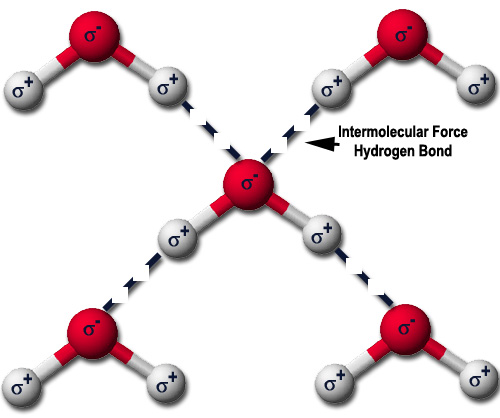
In this diagram, the solid connections are chemical bonds and the dashed connections are intermolecular forces. The bonds hold the water molecules together, and intermolecular forces make all of the water molecules stick together.
Types of Intermolecular Forces:
- Hydrogen Bonding and Dipole-Dipole attractions: happens when a molecule is polar (has +/- ends) and its opposite ends attract. These are strong intermolecular force, but simple hydrocarbons don’t have them because they aren’t polar.
- London Dispersion Forces: happens in simple hydrocarbons and other non-polar molecules; momentary charge differences cause momentary attractions. Weaker than hydrogen bonding, but still affects viscosity, boiling point, and density. Bigger molecules have stronger London Dispersion Forces.