In General Chemistry, we use the word “molecules” a bit too loosely. This keeps our lives simpler as you learn the difference between atoms and molecules… but unfortunately, it is wrong in some cases.
If you want to know why the compound making up table salt, for example, is not made of molecules, read on.
In compounds that actually are made of molecules, that means their atoms exist in separate little groups that are bonded together. Those are called molecular compounds.
Here is a diagram showing water, H2O, which is a molecular compound:
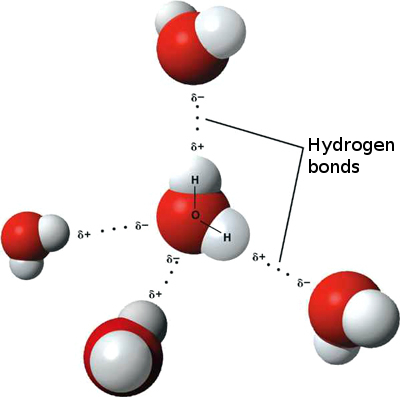
You can see groups of atoms that have two hydrogen (white) and one oxygen (red). They are distinct from the other molecules. It’s true that those molecules are attracted to each other (the dotted lines represent those weaker attractions), but you can still see the separate groups and see which atoms belong to which molecule.
Ionic compounds are not made of molecules. All of their atoms are connected together as one big, three dimensional pattern.
Here’s a model of what Na2O (an ionic compound) looks like:
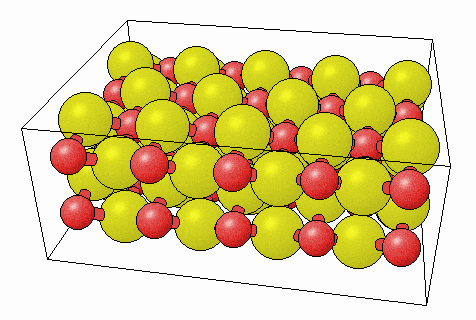
This is a crystalline solid, also known as a lattice solid. Again, that means the atoms are all connected in one big pattern.
Notice that the atoms are not grouped into sets of two sodium (Na) atoms and one oxygen atom. However, the element ratio is still there: you can see two sodium atoms (yellow) for every one oxygen (red). But we see no separated groups – no molecules.
Ionic compounds are made of ions (the individual charged atoms or groups of atoms), not molecules.
If we are talking about a balanced chemical equation that includes, for example, 3 Na2O, we can say that three formula units of Na2O are involved in the reaction. The formula unit is the simplest ratio of an ionic compound.
The moral of this story is that if you ever become a professional who deals with scientifically literate colleagues, don’t refer to “molecules” of an ionic compound in polite company.