“Periodic” means having a regular, repeating pattern.
In LMHS chemistry classes, we like to use this Periodic Table for paper copies.
Check out Dynamic Periodic Table at ptable.com
Each element box has the following info:

The elements are listed in order of their Atomic Number, which is their number of protons. Hydrogen has one proton, helium has two protons, and so on.
Horizontal Rows are called Periods on the Periodic Table.
Vertical Columns are called Families or Groups on the Periodic Table. Families are groups of similar elements – they often have similar chemical and physical properties.
There’s another note page for Element Families.
This note page shows where metals, nonmetals, and metalloids are found on the table.
If the elements are arranged in order by atomic number, a pattern does emerge. Every so often, an inert gas is followed by a reactive metal as shown below.
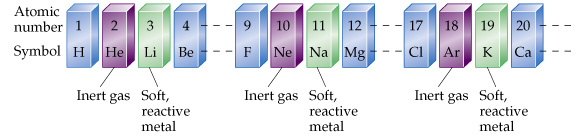
Many other patterns can be found within the Periodic Table if one looks at things like ion charges, boiling points, and other properties of the elements. For example, electronegativity (ability to attract electrons) tends increase as you move across the table from left to right, and it decreases down as you move down the table.
See Element Properties and Periodic Trends for more info on those patterns.