Atoms are made up of three smaller subatomic particles: protons, neutrons, and electrons.
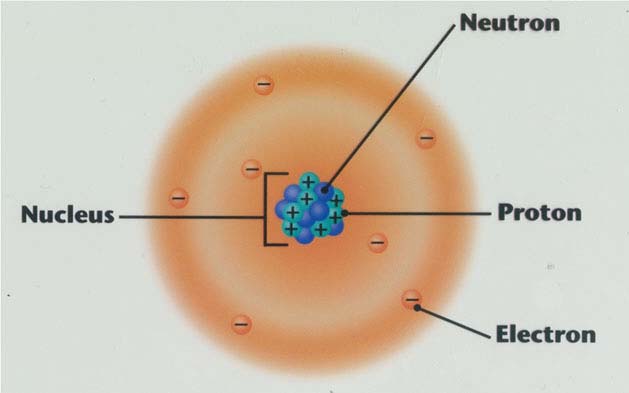
Nucleus: the tiny, dense, positively charged center of an atom
•protons and neutrons are in the nucleus
•makes up over 99.9% of an atom’s mass
•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape
Electron Cloud: region around the nucleus where electrons are found
•No definite boundary; atom’s radius is defined as the space where electrons spend 90% of time
•electrons do not follow nice orbits around a nucleus the way planets orbit a star; they are much weirder than that.
•electrons have properties of both waves and particles, which leads to some strange behavior. For example, a single electron is a wave-particle that can interfere with itself, because it behaves as if it exists as multiple waves in different places at the same time.
Electrical Charge
•protons are positively charged; since protons are in the nucleus, that means the nucleus is positive
•neutrons are neutral (no charge)
•electrons are negatively charged
•atoms are neutral, because they have the same number of protons and electrons; total charge adds up to zero
•atoms can become ions by gaining or losing electrons; ions have a non-zero charge because they have different numbers of protons and electrons.
Mass
•protons and neutrons have a mass of about 1 amu
•electrons have a mass of about 0.0005 amu, or 1/2000 the mass of a proton
Atomic Number:
•Number of protons in an atom
•Determines what element it is
•Example: an atom with 7 electrons is nitrogen (nitrogen is element #7 on the Periodic Table)
Mass Number:
•mass number = protons + neutrons
•total number of particles in the nucleus
•NOT included: electrons, because they have almost no mass
•must be an integer (whole number)
On the Periodic Table, you find the average atomic mass, which is not the same thing as mass number! Notice that the masses on the Periodic Table are decimals, and this is because they are averages.
Example: chlorine has an average atomic mass of 35.45; it’s a decimal partly because all of the chlorine in the earth’s crust is a mixture of two different forms of chlorine atoms – some (most) have a mass number of 35, while others have a mass number of 37. The average mass is somewhere in between.
Isotopes:
•Different forms of an element
•Not all atoms of a given element are identical to each other
•isotopes of an element have the same number of protons, but different number of neutrons.
•Example: nitrogen has two stable isotopes as described in the following table:
| Isotope Notation |
Protons | Neutrons | Mass Number |
| nitrogen-14 | 7 | 7 | 14 |
| nitrogen-15 | 7 | 8 | 15 |
The number in the isotope notation is the mass number.
Notice that both isotopes have 7 protons. As stated above, nitrogen always has 7 protons. As long as an atom has 7 protons, it could have any number of neutrons, and it would still be nitrogen.
Some isotopes are stable, while others are radioactive. Example: nitrogen-15 is stable, meaning it will stay the same indefinitely; nitrogen-16, by contrast, is a radioactive isotope that spontaneously turns into oxygen while emitting particles and energy.
Despite this difference in nuclear stability, nitrogen’s isotopes all have virtually identical chemical properties. This is true of nearly any element; hydrogen is the most notable exception.
Quick summary of some info about subatomic particles:
| proton | neutron | electron | |
| symbol | |||
| location | nucleus | nucleus | electron cloud |
| charge | +1 | 0 | -1 |
| mass | 1 | 1 | 0.0005 |