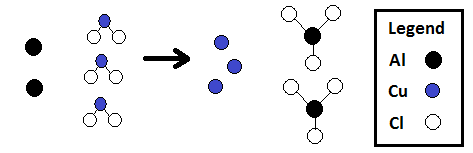
Using a chemical equation, you can figure out the number of atoms on each side OR the number of molecules on each side.
Atom Inventory (i.e. Atom Count)
![]()
| Reactants | Products | ||
| number of particles |
particle being counted |
number of particles |
particle being counted |
| 2 3 6 |
Al Cu Cl |
2 3 6 |
Al Cu Cl |
•Only list elements: notice that the “particle being counted” is always just a single element symbol with no subscripts
•Split up the molecules into individual elements
•The numbers in this chart match the number of circles of each color in the following diagram of this reaction:
•Both sides of an atom inventory need to be the same (same number of each type of atom); if the counts are different, that means the equation is creating or destroying atoms. The Law of Conservation of Mass says that can’t happen.
Molecule Inventory (i.e. Molecule Count)
![]()
| Reactants | Products | ||
| number of particles |
particle being counted |
number of particles |
particle being counted |
| 2 3 |
Al CuCl2 |
3 2 |
Cu AlCl3 |
•Here you’re counting particles again; however, the particles you’re counting are molecules. Molecules are made up of smaller particles atoms), so you could also say you’re counting groups of particles.
•Actually, “Al” and “Cu” are atoms, not molecules. When elemental substances like “Al” appear in reactions, you are stuck with atoms in your molecule count. This would probably be less confusing if it was called a “substance count” instead of “molecule count.” Basically, what you’re doing is counting how many particles of each substance participate in the reaction.
•Notice that we don’t end up with the same molecules on each side. Molecules are NOT a conserved quantity, which means that molecules CAN be created or destroyed, and you DO NOT have to end with the same number and type of molecules that you started with. Destroying a molecule doesn’t mean the matter is obliterated – it just means that the atoms disconnect from each other and rearrange to form different molecules.
•By the way, if you did have a reaction in which the molecule inventory was the same on both sides, it would mean that nothing happened. To take a real life example, here’s what happens if you mix sand (we’ll somewhat inaccurately call sand SiO2) and water (H2O):
![]()
You start with sand and water, and at the end you have… sand and water. Nothing happens. Chemistry is not very exciting when the reactant molecule count matches the product molecule count.