Parentheses indicate multiple identical groups of atoms; a subscript outside parentheses multiplies everything inside the parentheses.
Example: Pb(NO2)4
This could be drawn as follows:
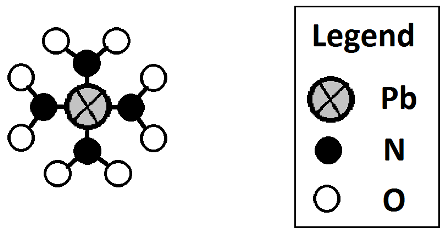
The (NO2) group is just this: ![]() . Four of those groups are shown because of the subscript 4 in (NO2)4. Notice that there is only one Pb atom – it is not multipled by the 4 since it is not inside the parentheses.
. Four of those groups are shown because of the subscript 4 in (NO2)4. Notice that there is only one Pb atom – it is not multipled by the 4 since it is not inside the parentheses.
If it was instead 3Pb(NO2)4 it would look the same, except you’d draw it three times:
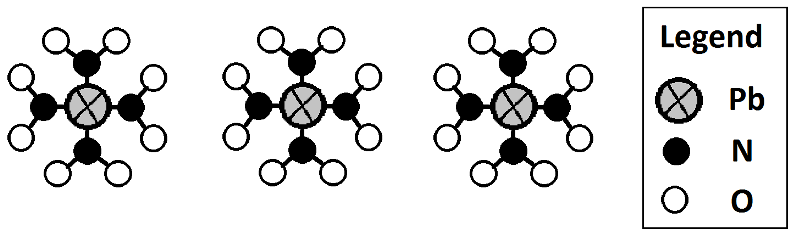
You can count the circles to find out how many atoms of each type are present in 3Pb(NO2)4. When you don’t have pictures, you multiply coefficients and subscripts together to get the atom counts.
Atom Count for 3Pb(NO2)4
Pb atoms:
3 molecules
x 1 Pb atom per molecule
= 3 Pb atoms
*(Pb is not multipled by 4, because it is not inside the parentheses)
N atoms:
3 molecules
x 1 N atom per group
x 4 groups of (NO2)
= 12 N atoms
“O” atoms:
3 molecules
x 2 “O” atoms per group
x 4 groups of (NO2)
= 24 “O” atoms