Example Chemical Equation:
![]()
“Two atoms of solid aluminum react with three molecules1 of aqueous copper(II) chloride to form three atoms of solid copper and two molecules of aqueous aluminum chloride.”
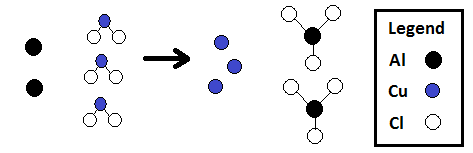
<– Previous: Atoms & Molecules / Next: Equation Symbols –>
1. For simplicity, this sentence is saying that the compounds in this reaction are made of molecules, but actually they’re not. Ionic compounds are not made of molecules.