The Periodic Table can sometimes be used to predict the properties of the elements.
As an example, let us predict the melting point of selenium (Se) in Kelvins (an absolute temperature scale that is useful in chemistry). We start by knowing the melting points of two other elements in the same family as selenium as follows:
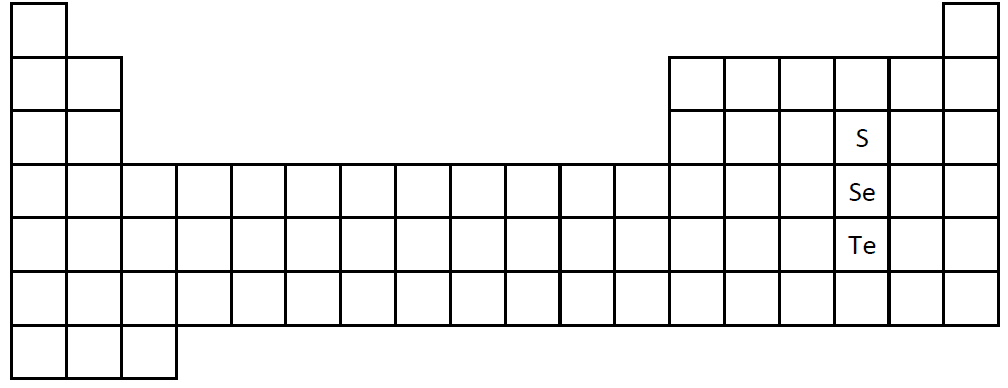
Melting Points:
sulfur (S): 388 K
selenium (Se): ???
tellurium (Te): 723 K
We can see that the melting point went up as we went down the table from S to Te. Our prediction is that selenium will be approximately in the middle of those two figures since Se is between S and Te on the table.
Our predicted melting point, then, is the average of 388 K and 723 K.
Predicted melting point of Se: 556 K
Actual melting point of Se: 494 K
Even though the predicted number is about 12% too high, that’s still pretty good! It’s way better than a random guess.